Drugs have been illegal in India for many years, but government permits a license for a business that deals with cosmetics and drugs
The drug is only legal if it is used for business in cosmetics and medicine. There are two departments like the Food Safety and Drug Department Administration of Uttar Pradesh that give drug licenses to prevent the manufacture and sale of sub-standard, fake medicines. In 1940, to implement the provision of the Drugs and Cosmetic Act. This article will let you know about procedures and other aspects to obtain a UP drug license for sale and for manufacture. You Can also learn about food and drug administration UP Services.
Documents Required for Uttar Pradesh Drug License for Manufacture
The following documents are prerequisites for applying for a Drug Manufacture License under the UP drug license:
1. Affidavit Attested by a Public Notary:
- Proprietor of the applicant firm for proprietorship firms
- All partners for partnership firms
- Person authorised by the Board of Directors for Private Limited or Limited Companies
- For Private Limited or Limited Companies, a copy of the minutes of the Board of Directors.
2. Additional Documents for Small Scale Units:
- Attested copy of the registration certificate from District Industries Centre.
3. Partnership Firm Documents:
- List of names and addresses of all partners.
- Partnership deed copy.
4. Address Proof for Authorised Applicant/Proprietor:
- Voter ID or Driving License.
5. Documents for Manufacturing Chemist Employed:
- Affidavit attested by a Public Notary
- Attested copies of educational and experience certificates
- Copy of the letter of approval by the State Drugs Controller
- Consent letter, appointment letter, or joining letter
- Medical certificate from a registered medical practitioner, including the eye fitness certificate
- Four photographs per applied UP drug license
- Address proof.
6. Documents for Analytical Chemist Employed:
- Similar documents as Manufacturing Chemist
- Attested copy of the consent of the Approved Testing Laboratory
- Three copies of the Plan of Premises showing all sections and dimensions
- Documentary proof of ownership or rental basis of the proposed premises
- Copy of rental agreement for rented premises
- Attested copy of ownership proof of rented premises from the property owner
- Copy of No Objection Certificate from Pollution Control Board
- For license renewal, attested copy of Air and Water Consent from the Pollution Control Board
- Copy of the water test report from a Government Approved Laboratory regarding its portability and freedom from pathogenic microorganisms.
7. Processing Time:
The license will be granted within 15 days upon online application submission.
Step-by-Step Procedure for UP Drug License Application
The Food Safety and Drug Administration Department of Uttar Pradesh has streamlined the process for citizens to apply for the drug licence UP through an online platform. Follow these steps for a hassle-free application:
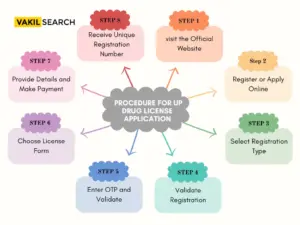
Step 1: Visit the Official Website
- Access the official website of Food Safety and Drug Administration, Uttar Pradesh.
- Click on ‘Online UP drug license Registration and Licensing System’ to enter the online application portal.
Step 2: Register or Apply Online
- If registered, click ‘Apply online for license.’
- For new users, click ‘Register’ at the top of the page, choosing either firm or technical person registration.
Step 3: Select Registration Type
- Specify the registration type: sales unit or manufacturing unit.
- Ensure a valid personal email ID and an active mobile number for registration.
Step 4: Validate Registration
- Validate the registration by entering the Aadhar number, triggering an OTP sent to the registered mobile.
Step 5: Enter OTP and Validate
- Enter the OTP received and click ‘Validate.’
Step 6: Choose License Form
- Select the appropriate license form based on your requirements.
Step 7: Provide Details and Make Payment
- Fill in the required details, complete the payment, and click ‘Submit.’
Step 8: Receive Unique Registration Number
- Upon successful registration, a unique registration number is generated, crucial for subsequent processing. Follow these steps diligently for a smooth and efficient application process.
License Condition
There are some conditions for getting a UP drug license which is listed below:
- The license will be given to those people only who have a practical experience in drug distribution.
- A registered pharmacist can directly and personally supervise any drug made by the license.
- The medicines used for treating animals should be labelled as “Not For Human Animal treatment only”.
- There should be inception by a registered pharmacist on the retail supply of the drugs prescribed by the medical practitioners.
- At the time of retail supply, it is very important to maintain the record of it.
- The licensed people should not sell or store drugs after their expiry date.
- This license provides a record of every purchase intended for sale or sold by retail.
- The UP drug license should not store any kind of drugs, that are used as a free sample for the medical professionals.
- If drugs are mentioned with Schedule H or Schedule X, they can only be valid for two years. These drugs should only be supplied only to registered medical practitioners, hospitals, dispensaries, and nursing homes when they have signed orders.
License For Selling In Uttar Pradesh
The drug sale license is applied for both wholesale and retail purposes to distribute drugs in India. This license will only be provided to those people who have an area of storing the drugs safely and have a pharmacy shop. License can be obtain with Food and drug administration UP.
Types of Drugs License Sale
There are different types of UP drug license sale as follow:
- For a Wholesale license, they need to fill the form 20B and 21B and need to be submitted at form 19.
- For a retail license, they need to fill the forms 20 and 21 and need to be submitted at form 19.
- For a Restricted drug licence UP, they need to fill the forms 20A and 21A and need to be submitted at form 19A.
- For Drugs specified in schedule X(wholesale), they need to fill the form 20G and need to be submitted at form 19C.
- For Drugs specified in schedule X(retail), they need to fill the form 20F and need to be submitted at form 19C.
Requirement For Drugs License in Uttar Pradesh
The following documents are required for getting a UP drug license.
A Written Statement Confirmed by Oath Should be Attested to Public Notary:
- If the owner of the business then adds a Sole proprietorship firm.
- If there are partners then attach a Partnership Registration.
- If the person is authorized by the Board of Directors of the Private Limited Company.
- There should be address proof of an authorized person or applicant like a copy of voter id, driver’s license.
- There should be a written document of the liable person for working every day and for any violation of rules related to drugs.
Documents Required if the Person is a Pharmacist:
- Written statements confirmed by oath should be attested to Public Notary.
- Attach all copies of educational qualifications certificates.
- Two photographs are mandatory.
- Documents related to address proof.
- The letter of appointment and joining.
Documents Requirement for Qualified People(Wholesale):
- All copies of educational certificates.
- Experience certificate in the written form by oath.
- Two photographs for each applied for UP drug license.
- If applicable attach both joining and appointment letters.
- There should be three plans submitted related to the proposed premises.
If expired drugs are stored for claiming a rebate from Income tax return will not be considered a crime until it is intended for sale.
Documents Requirement for Rental or Ownership Basis of the Proposed Premises:
- There should be a copy of the rental agreement in case of rental premises.
- If the person is the owner then attach the copy of ownership provided by the landlord.
There should be the invoice for the refrigerator attached with other documents. For more details, people can search the UPFDA on the internet.
Fee Structure
If people are interested in applying offline then they need to pay fees to the Government Treasury or Government Branch of the State Bank of India of the district where the license is required. Make sure to attach the challan with the application form.
- For a Wholesale License, the fees are ₹3000.
- For a Retail License, the fees are ₹3000.
- For a Restricted License, the fees are ₹1000.
- For Drugs specified in schedule-X(wholesale), the fees are ₹500.
- For Drugs specified in schedule-X(retail), the fees are ₹500.
Renewal of Drugs Sale License
For the renewal of Drugs sale license, they need to do a similar process which they did while taking the new license with some fees. The drug licence up fees for the renewal of the UP drug license are the same as for taking a new license.
Documents Requirement For Renewal Drugs License
- Attach a copy of the last renewal document.
- Testimony of Pharmacist with current Rental Agreement.
- Address proof of authorized people.
- There should be a written document of the liable person for working every day and for any violation of rules related to drugs.
Drugs Sale License for Manufacturer in Uttar Pradesh
There are different types of manufacturing licenses for manufacturers in Uttar Pradesh. Those are listed below:
- The drugs which are not mentioned in Schedule C, C(1), and X require to fill the form 24 and submit it.
- For Homeopathic medicine, form 24C is available.
- The drugs which are mentioned in Schedule X and not specified in Schedule C(1), and C require to fill the form 24F and submit it.
- The drugs which are mentioned in Schedule C and C(1) but not specified in Schedule X require to fill the form 27.
- .The drugs which are mentioned in Schedule C and C(1) on loan license but not specified in Schedule X require to fill the form 27A.
- The drugs are mentioned in Schedule C, C(1), and X require to fill the form 27B.
- The drugs which are manufactured for examination, test, or analysis, require to fill out form 30.
- The drugs which need approval for carrying out the test on cosmetics or raw materials used in manufacture on behalf of licensed people, need to fill out form 36.
Conclusion
The drug sale license is very important in Uttar Pradesh for starting your business in medical, hospitals, dispensaries, or small medical shops. There are online procedures also to get the UP drug license, you just need to visit the Food Safety and Drug Administration’s website. Documents requirements will be the same. In almost under 14 days you will receive your drug license if your documents are verified correctly.
FAQs on UP Drug License
What is a drug license?
A UP drug license is an official document issued by regulatory authorities, allowing individuals or entities to engage in activities related to the manufacture, sale, distribution, or storage of drugs and pharmaceuticals. It ensures compliance with safety and quality standards in the pharmaceutical industry.
What is the cost of a drug license in UP?
The cost of a UP drug license varies based on the type and scale of the pharmaceutical activities. Fees are structured differently for manufacturing, sale, or distribution licenses. It's advisable to check with the Food Safety and Drug Administration Department of Uttar Pradesh for specific and up-to-date fee details.
What is Form 20B & 21B?
Form 20B and 21B are specific application forms used for obtaining drug licenses in India. Form 20B is for the grant or renewal of a wholesale drug license, while Form 21B is for the grant or renewal of a retail UP drug license. These forms are essential components of the licensing process, capturing crucial information for regulatory purposes.
What documents are required for a UP drug license?
To obtain a drug license in Uttar Pradesh, you need the following documents: a cover letter, application form, key plan and site plan of the premises, proof of ownership or lease of premises,incorporation certificate , qualification proof of the applicant, appointment letter of the registered pharmacist or competent person, and an affidavit of non-conviction of the applicant.
What is Form 28 in pharma?
Form 28 in the pharmaceutical industry is an application form used for the grant or renewal of a license to manufacture drugs or cosmetics. It ensures compliance with the Drugs and Cosmetics Act, 1940, and certifies that the applicant meets the necessary standards and regulations for manufacturing.
How can I check my drug license online in India?
To check your drug license online in India visit the official website of the Central Drugs Standard Control Organization (CDSCO) or the respective State Drug Control department. Navigate to the Drug License section and enter your application number or license number to view the status or details of your license.
How many types of drug licenses are there?
There are primarily three types of drug licenses in India: Retail Drug License: Issued to pharmacies and chemists for the sale of drugs directly to customers. Wholesale Drug License: Granted to businesses that supply drugs in bulk to retailers or other wholesalers. Manufacturing Drug License: Required for the production of drugs and cosmetics. Each type of license ensures adherence to safety, quality, and regulatory standards specific to the nature of drug distribution and manufacturing.
How long is a drug license valid for?
A drug license in India is typically valid for five years from the date of issue. However, the validity can vary depending on state regulations and the specific type of license. License holders must apply for renewal before the expiration date to continue operations without any legal interruptions.
What is 21C in pharmacy?
Section 21C of the Drugs and Cosmetics Act, 1940, pertains to the standards for blood banks. It lays down the criteria and requirements for establishments involved in the collection, storage, processing, and distribution of blood and blood components to ensure safety and efficacy in blood transfusions and related services.
What are the 7 drug categories?
The seven types of drugs categories are Analgesics, Antibiotics, Antidepressants, Antihistamines, Antihypertensives, Antivirals and Diuretics







